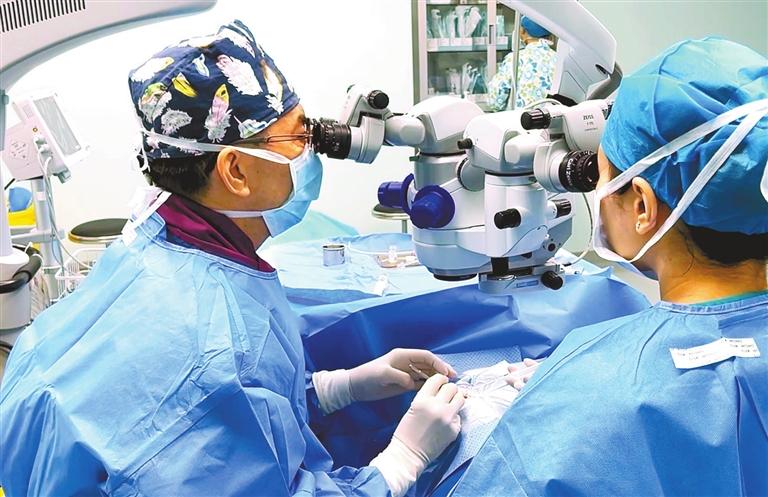
Debra Li debra_lidan@163.com A PATIENT successfully received a Loong Crystal PR Phakic intraocular lens (IOL) implant, the first homegrown IOL product designed and manufactured in China, during a surgery at Shenzhen Bright Eye Hospital in Nanshan District on Saturday. Developed by Beijing-based Eyebright Medical Technology Co. Ltd., the Loong Crystal PR was approved by China’s National Medical Products Administration in January and entered the domestic market in February. Song Beijia, a marketing official with Eyebright Medical Technology, compared her company to Huawei in the field of ophthalmic devices. “Our company has invested heavily in research and development since its founding in 2010, and our intraocular lens products that treat cataracts currently occupy one-third of the domestic market," Song told Shenzhen Daily during an interview Saturday. "The Loong Crystal PR, approved after nearly 200 successful implants in more than five years of clinical trials, has better performance than imported products and is priced at 2,000 yuan (US$280) higher than imports,” she continued. “As evidenced by Huawei phones, China-made products need not be cheap or inferior to imports,” she added. Designed for the treatment of adult patients, the IOL, available in 10 sizes, provides effective correction or reduction of myopia ranging from -3.25D to -18.00D, offering a tailored solution for individuals with varying degrees of myopia. Constructed from an advanced balanced acrylic material, the IOL incorporates a zero spherical aberration design with a large optical zone and a double-concave stable arch height structure, ensuring better vision in dark environments. Liu Quan, head of the hospital’s corneal-refractive laser surgery team, performed the surgery on a 33-year-old woman surnamed Jiang. Suffering from myopia since junior high school, the patient had -5.00D myopia in both eyes and astigmatism in the right eye before the surgery. With thin corneas and severe dry eye syndrome, Jiang could not receive other refractive surgeries like LASIK. She had planned to receive double implants of Staar Surgical’s Implantable Collamer® Lens (ICL), but pre-surgery medical exams found the arch height (the distance between a patient’s natural lens and the implanted lens) in her left eye would not be safe enough for an ICL implant. “The Loong Crystal PR is thinner than ICL, which makes it a perfect choice for Jiang,” Liu explained. Medical checks found the patient had normal vision in both eyes six hours after the surgery. Jie Jiangbing, founder and CEO of Eyebright Medical Technology Co. Ltd., is a top researcher in chemical and biomolecular engineering. He previously served as chief scientist with Advanced Medical Optics Inc., a U.S.-based leader in medical optics, before returning to China to start his company. Bright Eye Hospital is a listed medical service chain operating 35 hospitals in 24 Chinese cities, including Shenzhen and Guangzhou. | 
